Cell-to-cell communication
Bacteria that regulate gene expression in response to changes in cell density are said to engage in quorum sensing. To synchronize their programs of gene expression during the growth of a colony, and perform collective tasks such as resisting antibiotics or inducing virulence factors during infection of a host, quorum-sensing bacteria need to glean as much information as possible about their cell density.
Despite many experimental studies, the biophysical limits on quorum-sensing information transfer remain unclear. Biochemically, a hallmark of quorum-sensing systems is feedback, be it within the intracellular biochemical pathways or mediated via diffusible molecules at the level of the colony. We employ a biologically relevant model for quorum sensing with feedbacks to quantify the information available to a bacterium during the growth of a colony. Specifically, we consider the quorum-sensing system as an information channel and optimize the mutual information between cell density and the expression of a monitor gene by varying the functional form of feedback, i.e. by varying the encoding scheme of the channel.
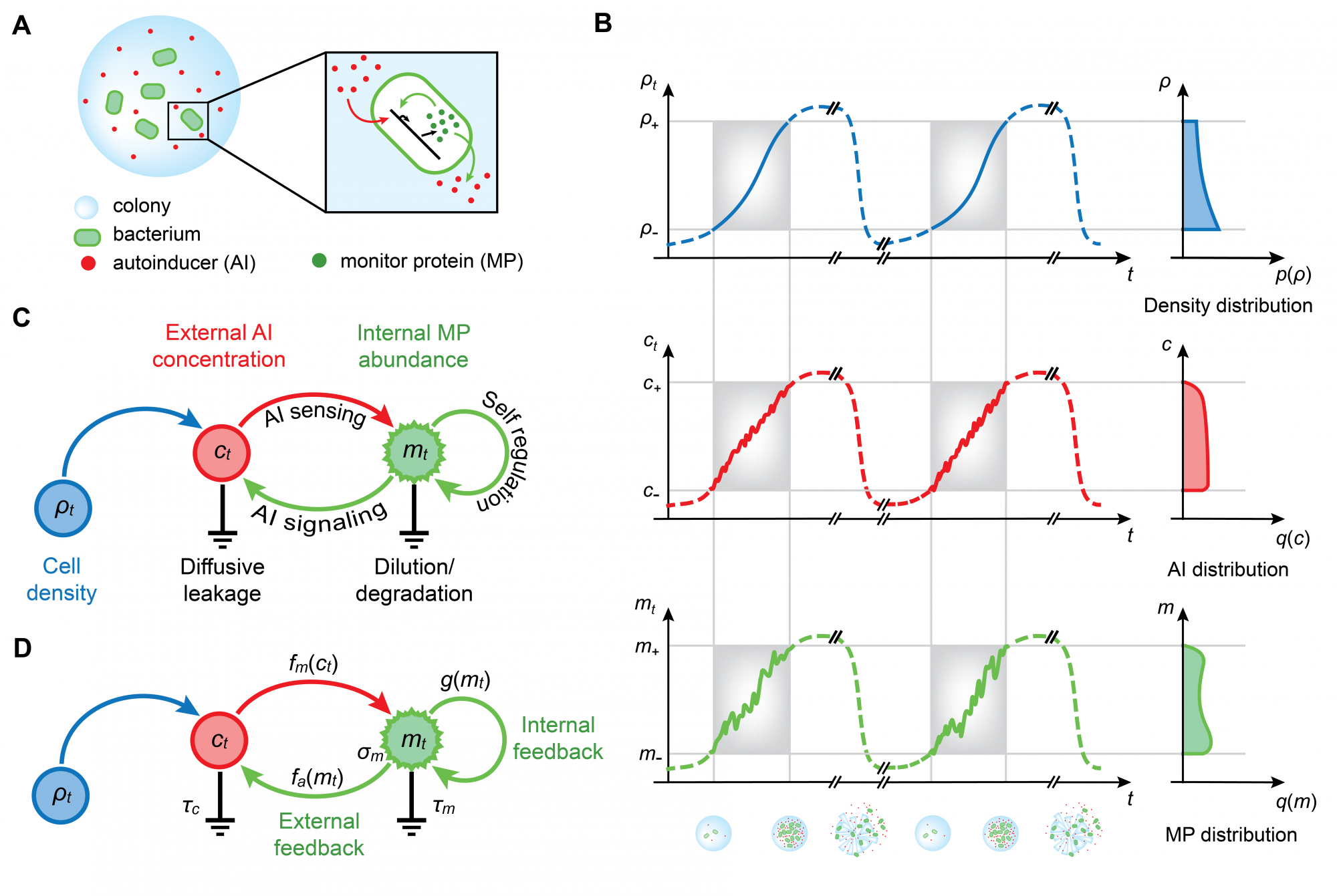
Our rigorous approach yields a physically interpretable expression for the optimal quorum-sensing information transfer, bypassing the need to infer unknown biological parameters. Moreover, our theoretical analysis characterizes the dual role of feedbacks in promoting information transfer: (1) At the population level, feedbacks allow the detection channels of individual cells to operate at their information capacity. (2) Intracellularly, feedbacks increase the capacity of detection channels by reducing the effective timescale of the quorum-sensing response. Finally, we contrast the information capabilities of distinct feedback mechanisms observed in quorum sensing—regulation by transcription factor or by small RNAs—and suggest complementary roles when both mechanisms are present in the same circuit. This work extends the information theoretical concept of histogram equalization—originally developed in the context of neuroscience—to include the benefit of feedbacks to information transfer in biological systems, a general result that is applicable well beyond quorum sensing.
Metabolic diversity
Bacterial diversity is ubiquitous. Metagenomics has revealed hundreds of bacterial species in almost all microbiota. Shotgun sequencing has begun to unveil the biochemical networks at work in these communities. In a few well-studied cases, bacterial communities have been observed to coordinate their metabolic fluxes, thereby engaging in division of labor. In principle, bacteria can divide tasks to reap the benefits of specialization, as in human economies. However, owing to our lack of knowledge of gene and protein distributions in individual cells, the benefits and stability of an economy of bacterial specialists are far from obvious.
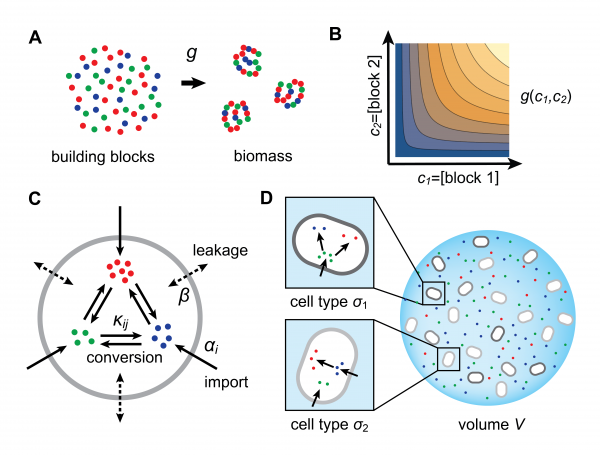 We employ a physical model for resource-limited growth to understand the stable emergence of division of labor in a community of metabolically competing bacteria. Specifically, we consider that biomass results from the assembly of building blocks and we explicitly model the fluxes associated with the metabolic processing of these building blocks, including enzyme-mediated import and conversion. This approach shows that population dynamics generally leads to the coexistence of different metabolic types, which are defined by specific distributions of enzymes with a budget constraint. The figure above recapitulates the essential tenets of our model.
We employ a physical model for resource-limited growth to understand the stable emergence of division of labor in a community of metabolically competing bacteria. Specifically, we consider that biomass results from the assembly of building blocks and we explicitly model the fluxes associated with the metabolic processing of these building blocks, including enzyme-mediated import and conversion. This approach shows that population dynamics generally leads to the coexistence of different metabolic types, which are defined by specific distributions of enzymes with a budget constraint. The figure above recapitulates the essential tenets of our model.
Importantly, the metabolic strategies selected in bacterial consortia—and thus metabolic division of labor—can be characterized in relation to the external availability of steadily-supply building blocks. The figure below illustrates the emergence of microbial consortia for representative supply rates in the case of three building-blocks.
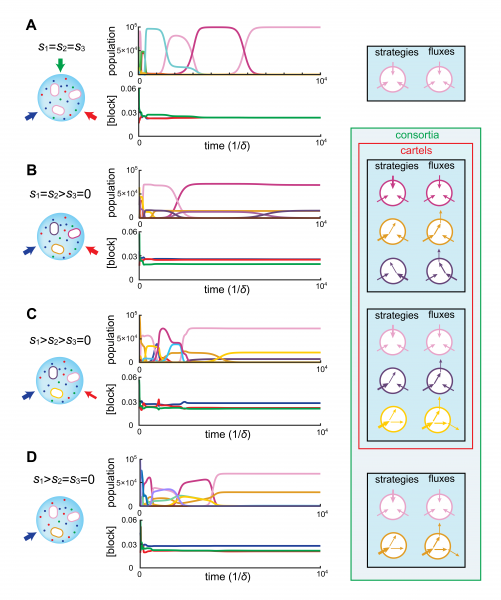
Exploiting this characterization of division labor, we establish that consortia act as cartels, whereby population dynamics pins down resource concentrations at values for which no other metabolic type can invade. Moreover, our analysis supports that at steady supply, cartels of competing strategies automatically yield maximum biomass, thereby achieving a collective optimum.
These results can be interpreted within the framework of optimal transport-network theory. To be stable against invasion by metabolic variants, members of consortia are necessarily co-optimal cell types at given external resources concentration—no other cell type can outgrow them. Because internal metabolic cycles are inherently wasteful at steady state, the requirement for optimal cell growth imposes a simple tree-like structure to cellular metabolic network. In a population of cell types, these individually optimal metabolic networks establish a transport network whose overall task is to convert building-blocks into biomass. Division of labor is selected because at given building-block supply, the maximum rate of biomass production is generally achieved for networks of distinct co-optimal strategies.